This is a flow-chart I put together, that may go in the documentation for the Health Research Council data monitoring committee. Obviously the questions in boxes are simplified and would need expansion in the text.
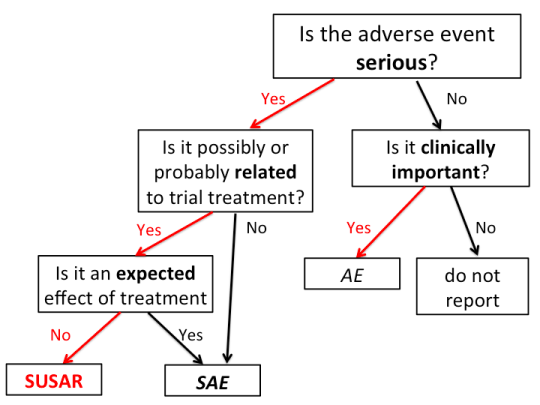
Most of the work of the data monitoring committee, and the work that the study does on our behalf, is concentrated at the twice-yearly meetings. Some things, though, can’t safely be left to accumulate for six months, so there’s urgent reporting of sufficiently noteworthy clinical events to a clinician on the data monitoring committee.
SUSAR stands for Suspected Unexpected Serious Adverse Reaction; you can see why we use the acronym. A SUSAR means: a Seriously Adverse thing happened to someone; there was some reason to attribute it to the treatment, so it’s a Suspected Reaction; and it was Unexpected, so participants weren’t warned about the possibility before giving consent.
It’s pretty standard around the world that SUSARs are reported urgently. The problem with just doing this with SUSARs is that the Suspected and Unexpected aspects are a bit subjective. For example, it might be that the severity is Unexpected, even though qualitatively the Adverse Reaction is Expected. Or it might be that the trial investigators don’t Suspect a connection to treatment, but a more paranoid person might. For that reason, it’s reasonably common for all Serious Adverse Events to be reported urgently, even if they aren’t Unexpected or aren’t Suspected Reactions.